Category: Uncategorized
-
Understanding the Critical Role of Post Market Surveillance in the Medical Device Industry

Launching your medical device into the market is just the beginning, not the conclusion, of your groundbreaking journey. Despite the immense effort poured into development, regulatory approvals, and manufacturing, it’s crucial to recognize that the journey doesn’t conclude with market entry. Beyond the initial milestones, regulatory bodies mandate an ongoing commitment known as post-market surveillance…
-
MedTech News Briefs | January 29th, 2024

Monday, January 22nd Tuesday, January 23rd Wednesday, January 24th Thursday, January 25th Friday, January 26th Sources
-
MedTech News Briefs | January 22nd, 2024

Monday January 15th Tuesday January 16th Wednesday January 17th Thursday January 18th Friday January 19th Sources
-
Clinical Trials for Your Medical Device: An Introduction
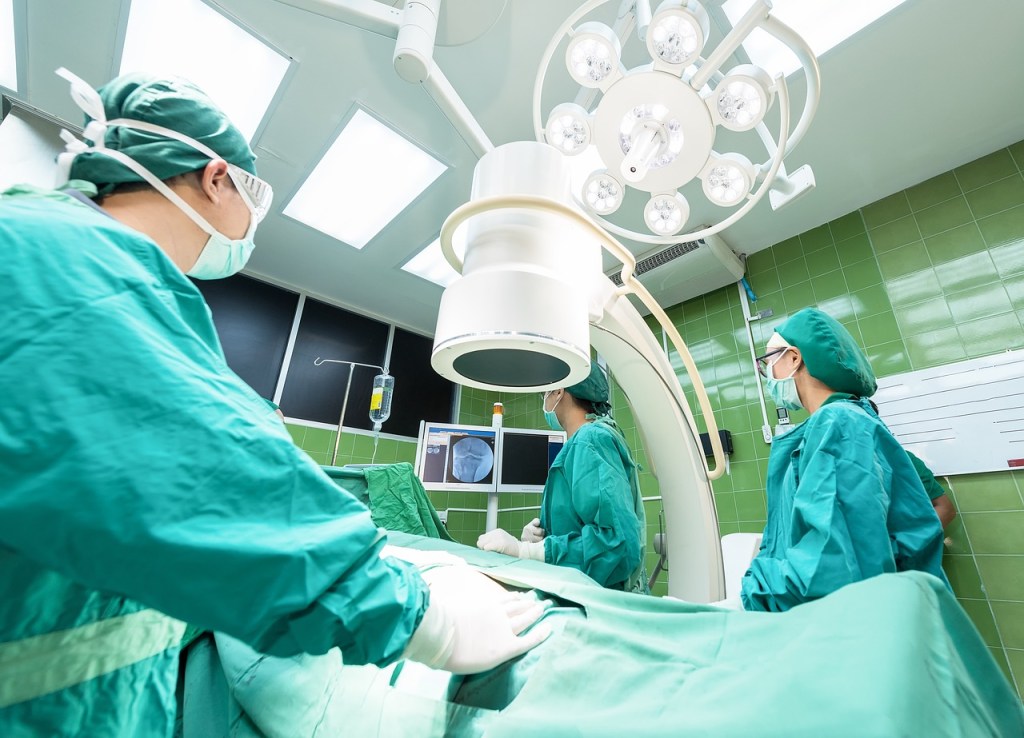
Being a part of medical device development isn’t easy. It requires a lot of failure and trials. One trial that every developer will face is that of clinical trials. Clinical trials helps you to evaluate the safety and efficacy of interventions and is a requirement by regulatory bodies. In this article we dive into what…
-
MedTech News Briefs | January 15th, 2024

January 8th, 2024 January 9th, 2024 January 10th,2024 January 11th, 2024 January 12th, 2024 Sources
-
Polymers for Medical Devices: Navigating Challenges and Embracing Solutions

In the complex world of medical device engineering, the use of polymers presents both challenges and transformative solutions. The demands are high: materials must endure rigorous sterilization procedures, interact well with the human body, and maintain mechanical performance. This article explores the challenges of using polymers in medical devices and the practical benefits they bring…
-
MedTech News Briefs | January 8th, 2024

Tuesday January 2nd Wednesday January 3rd Thursday January 4th Friday January 5th Sources:
-
Navigating the CE Marking Process: An Introduction for Medical Device Startups

In the medical device industry one crucial marker stands out – the CE marking. This insignia is not just a symbol; it is a gateway to the expansive European Union (EU) market, signifying adherence to rigorous standards for safety, health, and environmental protection. For medical device startups eyeing market expansion, understanding the intricacies of the…
-
MedTech News Briefs | January 2nd, 2024

Happy 2024 Everyone! Due to last week being a holiday week this week’s news post is shortened. Sources
-
2024 for Think MedTek

Happy New Year and welcome to 2024 everyone! Around the end of each year and as we approach a new year I like to sit down and take time to think about what I want to do for each year. For me I try to include several areas of my life including family, fitness, faith,…
